
My research projects include both heterogeneous and homogeneous electrocatalysis for water splitting and CO2 reduction. I am especially interested in developing electrochemical catalysis system to produce chemical feedstock with green electricity.
Projects at Caltech (2021-present)
- Tuning Product Selectivity of CO2 Reduction By Controlling Electrode/Electrolyte Interface
At LiSA (Liquid Sunlight Alliance), I am working on improving product selectivity and inhibiting competing hydrogen evolution reaction of electrochemical CO2 reduction reaction via electrolyte engineering. By applying water-in-salt electrolyte, we can increase the gas product selectivity for C2H4 over CO and CH4 simultaneously on Cu gas diffusion electrode. We are currently working with collaborators to investigate the Cu/water-in-salt electrolyte interface and understand the origin of the high C2 gas product selectivity. Besides that, I am also developing aqueous-nonaqueous mixed electrolytes to control proton delivery pathways, and control the mass transport of CO2 and reaction intermediates to tune the product distribution.
Projects at OSU (2019-2021)
- Paddlewheel Dirhodium Catalyst for H2 Evolution
When working at OSU, I was mostly focused on studying the reaction mechanism of a paddlewheel dirhodium electrocatalyst for hydrogen evolution reaction (HER). I elucidated a new electrochemical strategy of pre-catalysis proton relay insertion on HER electrocatalyst via electrochemistry analysis, CV simulation and DFT calculation (S. Lin, et al., J. Am. Chem. Soc. 2022, 144, 44, 20267).
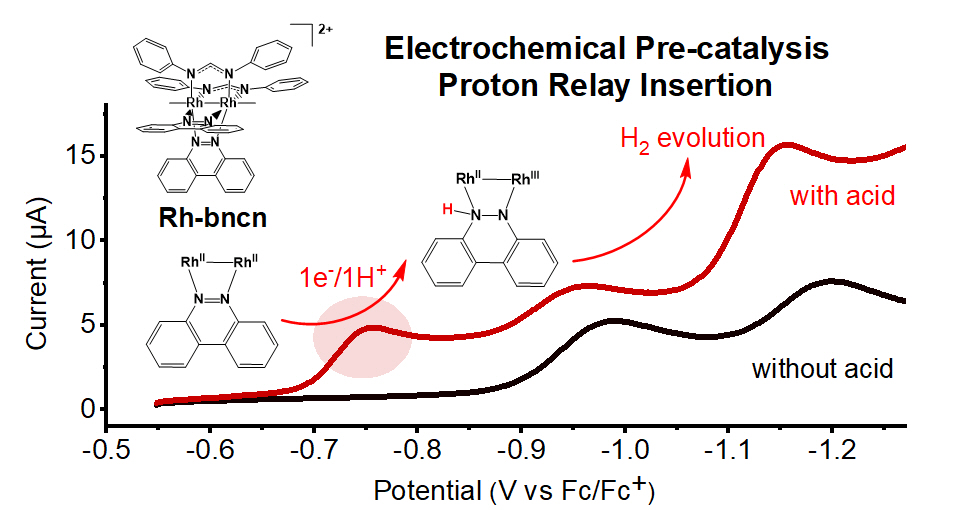
My other projects included exploring ways of tuning the selectivity of CO2 reduction catalysts, as well as understanding the impact of the molecular structure on the photochemistry behavior of dirhodium photosensitizer (S. Lin, et al., Chem. Eur. J. 2021, 27, 5379).
Projects at VT (2013-2019)
- Water Oxidation Catalyzed by MOF
In this study, I incorporated a molecular water oxidation catalyst [Ru(tpy)(dcbpy)OH2]2+ (tpy=2,2′:6′,2′′-terpyridine, dcbpy = 5,5′-dicarboxy-2,2′-bipyridine) into a porous solid-state metal organic framework UiO-67. The resulting heterogeneous water oxidation catalysts, RuTB doped MOFs (RuTB-UiO-67) exhibited a single-site reaction pathway with kinetic behavior in consistent with the homogeneous RuTB catalyst. We demonstrated that RuTB is reactive throughout the MOF particles (S. Lin, et al., ChemSusChem, 2018, 11, 464-471).

To circumvent the need for sacrificial electron acceptors in chemical water oxidation reaction, RuTB-UiO-67 MOF thin films grown on conducting FTO substrate (RuTB-UiO-67/FTO) were synthesized to test their catalytic activity towards electrochemical water oxidation. I found that the electrochemical behavior of RuTB-UiO-67/FTO was consistent with homogeneous RuTB by various electrochemistry study and in-situ X-ray absorption spectroscopy characterization. The highest electroactive catalytic site coverage on the electrode was 120 times higher than that of a full packing monolayer of the same molecular catalyst (S. Lin, et al., ChemSusChem, 2017, 10, 514-522).
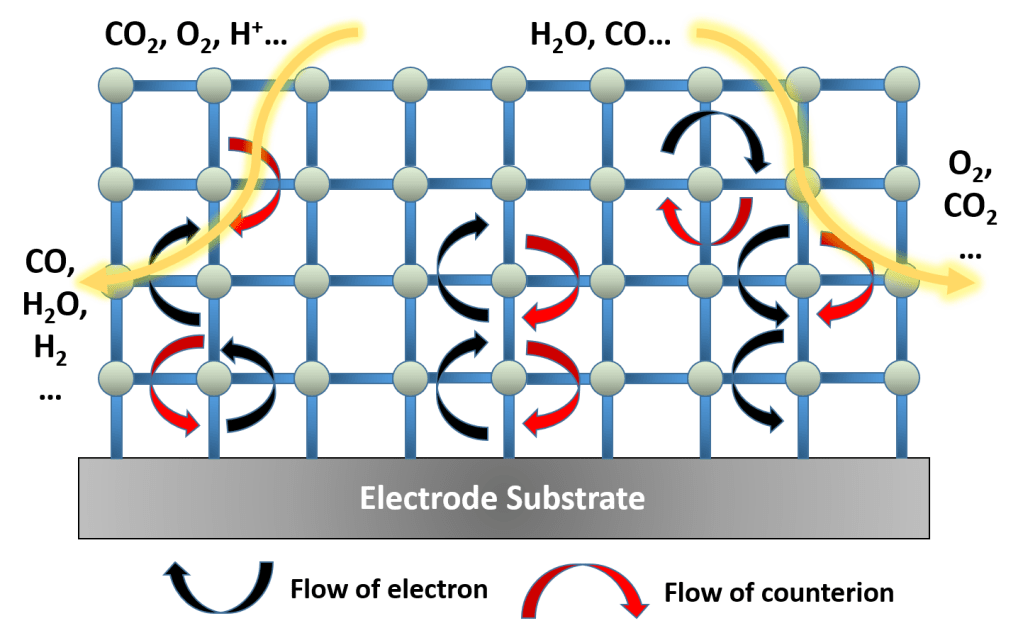
- Charge transfer in MOF
In the previous work, I demonstrated the homogeneous distribution of electrochemical active sites throughout the MOF thin film. Diffusion controlled redox hopping was attributed to be the main charge transfer pathway during catalysis. Redox hopping plays an important role in determining the rate of electrocatalysis by MOF, besides the intrinsic reactivity of the incorporated catalyst, and the mass transfer rate of reactants/products (S Lin, et al., Chem. Commum, 2018, 54, 6965-6974).
- Photo-electrochemcial Alcohol Oxidation
In order to pursue photo-induced water splitting system, I chose photoelectrochemical alcohol oxidation as the preliminary-stage study towards the more challenging goal, photoelectrochemical water oxidation. A mixed linker method was introduced to incorporate both the catalysts (RuTB) and photosensitizer (RuB, [Ru(bpy)2(dcbpy)]2+) into the same MOF scaffold grown on the surface of TiO2/FTO substrate. Electron transfer processes of the photosensitizer and the catalyst doped UiO-67 MOF were investigated with transient absorption spectroscopy analysis. Extension of charged separated states of the catalyst which facilitate the catalysis reaction was observed. We proposed that it is due to cooperative effect in the mixed linker MOF (S. Lin, et al., Faraday Discuss., 2021, 225, 371-383).
